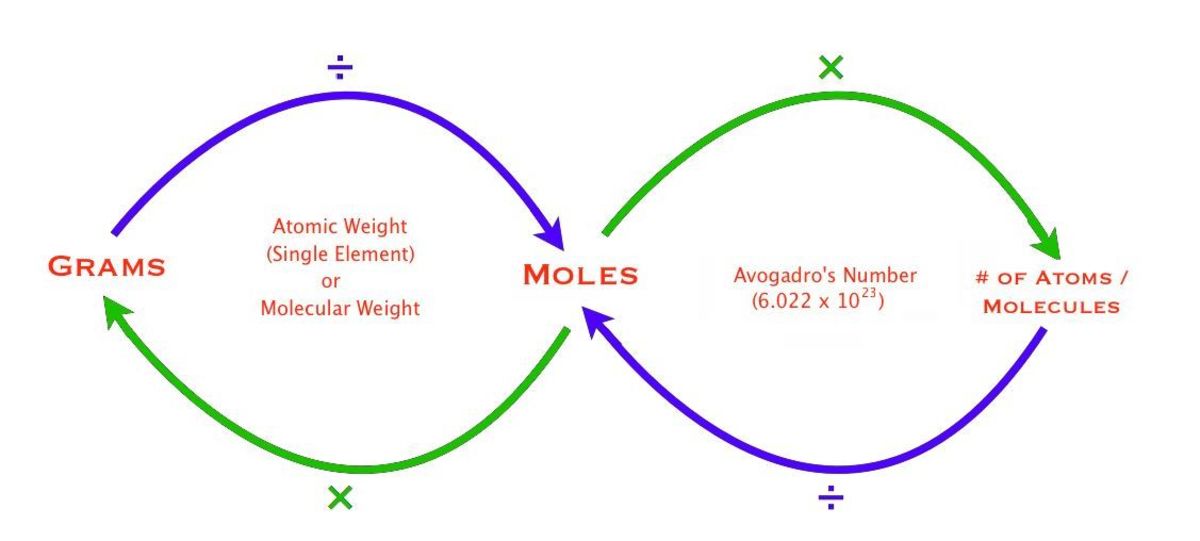
To convert from moles to atoms, multiply the molar amount by Avogadro’s number. To convert from atoms to moles, divide the atom amount by Avogadro’s number (or multiply by its reciprocal). Mole: The amount of substance of a system that contains as many elementary entities as there are atoms in 12 g of carbon-12. Converting moles to atoms is as simple as multiplying the number of moles by the 6.022. 10^23 because by definition that is what a mole represents. Moles to Atoms Formula. The following formula is used to convert the total moles to total atoms. A = M. 6.0221415.10^23. Where A is the number of atoms; M is the number of moles; Moles to Atoms Definition. Converting moles to atoms is as simple as multiplying the number of moles by the 6.022. 10^23 because by definition that is what a mole represents.
Even with a good word-processing program, having to click on an icon to get a superscript, and then remembering to click off after you type the number can be a real hassle. If we did not know of moles, and instead just knew of numbers of atoms or molecules (big numbers that require lots of superscripts), life would be much more complicated; we would certainly make more typing errors.
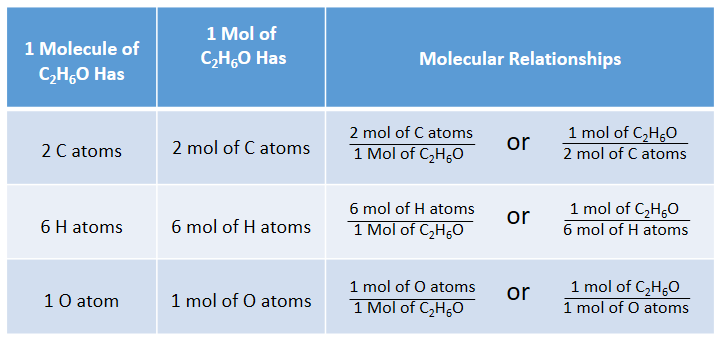
Conversions Between Moles and Atoms
Conversions Between Moles and Number of Particles
Using our unit conversion techniques, we can use the mole label to convert back and forth between the number of particles and moles.
Example (PageIndex{1})
The element carbon exists in two primary forms: graphite and diamond. How many moles of carbon atoms is (4.72 times 10^{24}) atoms of carbon?
Solution
Step 1: List the known quantities and plan the problem.
Known
- number of (ce{C}) atoms (= 4.72 times 10^{24})
- (1) mole (= 6.02 times 10^{23}) atoms
Unknown
- (4.72 times 10^{24} = ?) atoms (ce{C})
One conversion factor will allow us to convert from the number of (ce{C}) atoms to moles of (ce{C}) atoms.
Step 2: Calculate.
[4.72 times 10^{24} : text{atoms} : ce{C} times frac{1 : text{mol} : ce{C}}{6.02 times 10^{23} : text{atoms} : ce{C}} = 7.84 : text{mol} : ce{C}]
Step 3: Think about your result.
The given number of carbon atoms was greater than Avogadro's number, so the number of moles of (ce{C}) atoms is greater than 1 mole. Since Avogadro's number is a measured quantity with three significant figures, the result of the calculation is rounded to three significant figures.
Suppose that you want to know how many hydrogen atoms are in a mole of water molecules. First, you need to know the chemical formula for water, which is (ce{H_2O}). There are two atoms of hydrogen in each molecule of water. How many atoms of hydrogen are in two water molecules? There are (2 times 2 = 4) hydrogen atoms. How about in a dozen? In that case, a dozen is 12; so (12 times 2 = 24) hydrogen atoms in a dozen water molecules. To get the answers (4 and 24), you multiply the given number of molecules by two atoms of hydrogen per molecule. So, to find the number of hydrogen atoms in a mole of water molecules, the problem can be solved using conversion factors:
[1 : text{mol} : ce{H_2O} times frac{6.02 times 10^{23} : text{molecules} : ce{H_2O}}{1 : text{mol} : ce{H_2O}} times frac{2 : text{atoms} : ce{H}}{1 : text{molecule} : ce{H_2O}} = 1.20 times 10^{24} : text{atoms} : ce{H}]
The first conversion factor converts from moles of particles to the number of particles. The second conversion factor reflects the number of atoms contained within each molecule.
Example (PageIndex{2})
Sulfuric acid has the chemical formula (ce{H_2SO_4}). A certain quantity of sulfuric acid contains (4.89 times 10^{25}) atoms of oxygen. How many moles of sulfuric acid is the sample?
Solution:
Step 1: List the known quantities and plan the problem.
Known
- (4.89 times 10^{25} = ce{O}) atoms
- (1) mole (= 6.02 times 10^{23}) molecules (ce{H_2SO_4})
Unknown
- (text{mol}) of (ce{H_2SO_4}) molecules
Two conversion factors will be used. First, convert atoms of oxygen to molecules of sulfuric acid. Then, convert molecules of sulfuric acid to moles of sulfuric acid.
Dicom download for mac. Step 2: Calculate.

[4.89 times 10^{25} : text{atoms} : ce{O} times frac{1 : text{molecule} : ce{H_2SO_4}}{4 : text{atoms} : ce{O}} times frac{1 : text{mol} : ce{H_2SO_4}}{6.02 times 10^{23} : text{molecules} : ce{H_2SO_4}} = 20. : text{mol} : ce{H_2SO_4}]
Step 3: Think about your result.
The original number of oxygen atoms was about 80 times larger than Avogadro's number. Since each sulfuric acid molecule contains 4 oxgyen atoms, there are about 20 moles of sulfuric acid molecules.
How Do You Convert Moles To Atoms
Summary
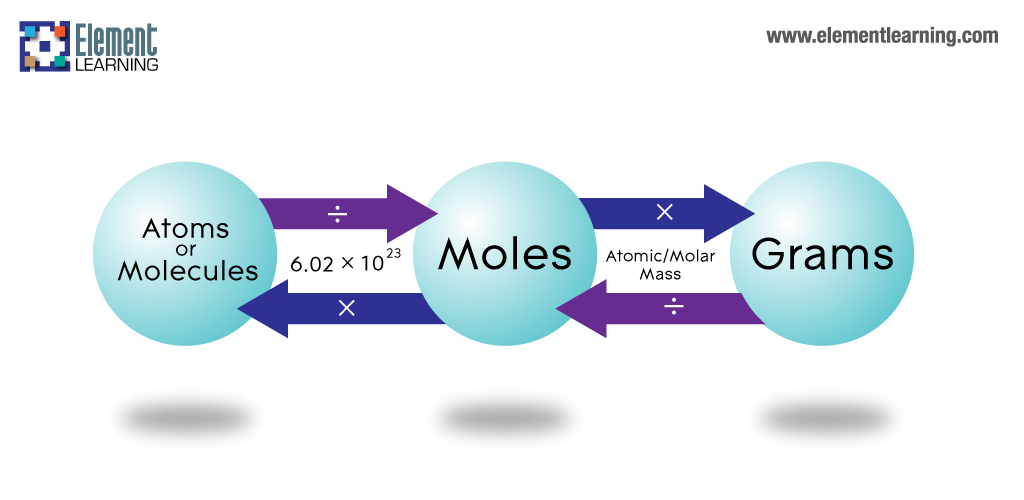
- Methods are described for conversions between moles, atoms, and molecules.
Contributors and Attributions
Moles To Particles Calculator
CK-12 Foundation by Sharon Bewick, Richard Parsons, Therese Forsythe, Shonna Robinson, and Jean Dupon.
