Key Difference – sp3d2 vs d2sp3 Hybridization
There are hypothetical structures known as orbitals in an atom in which electrons reside. Different scientific discoveries have proposed different shapes for these orbitals. Atomic orbitals can undergo a process known as hybridization. Hybridization of orbitals occurs in order to obtain suitable shapes required for chemical bonding. Hybridization is the mixing of atomic orbitals to form hybrid orbitals. sp3d2 and d2sp3 are such hybrid orbitals. The key difference between sp3d2 and d2sp3hybridization is that sp3d2 hybridization involves atomic orbitals of same electron shell whereas d2sp3 hybridization involves atomic orbitals of two electron shells.
CONTENTS
1. Overview and Key Difference
2. What is sp3d2 Hybridization
3. What is d2sp3 Hybridization
4. Similarities Between sp3d2 and d2sp3 Hybridization
5. Side by Side Comparison – sp3d2 vs d2sp3 Hybridization in Tabular Form
6. Summary
What is sp3d2 Hybridization

The remaining 4 unpaired electrons form the sp3d2 hybridization, which consists of 2 unpaired electrons in the 5p orbital and 2 others in the 5d orbital. A sigma bond is created in the process. Polarity in XeF4. The molecule XeF4 is a nonpolar molecule. As the geometrical structure of XeF4 is symmetric ie; square planar. The answer is option E that is the sp3d2 atomic hybrid orbital set accomates 6 electron domain. The number of electron domains shows by a set of hybrid orbitals that can be foun view the full answer Previous question Next question.
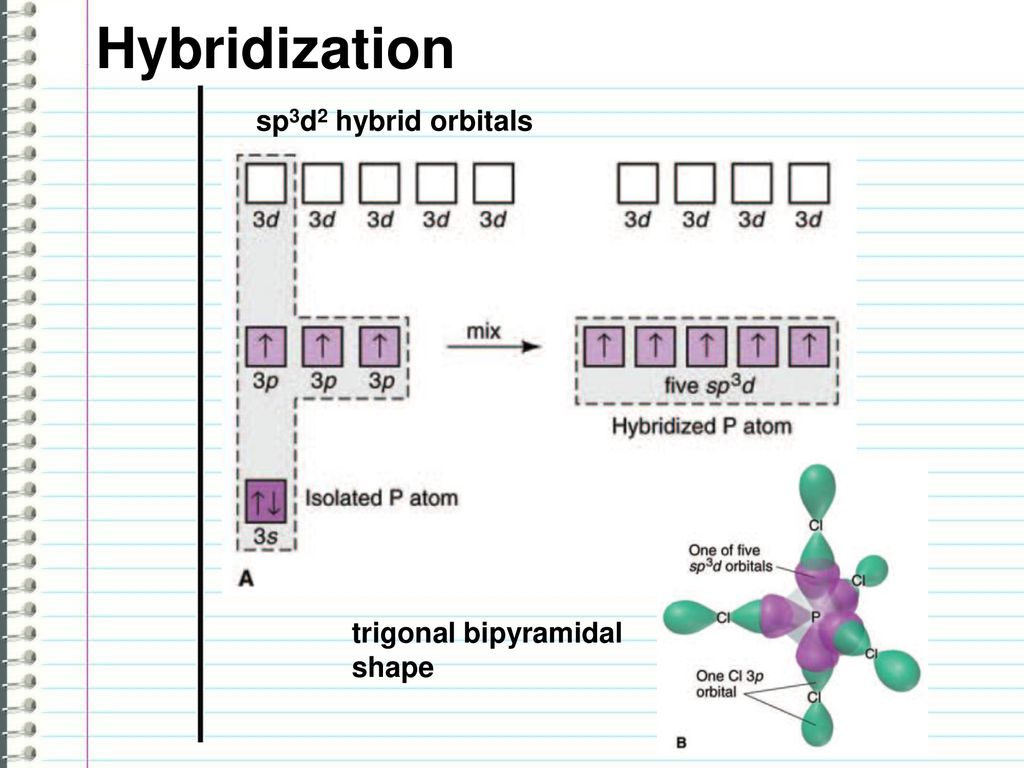
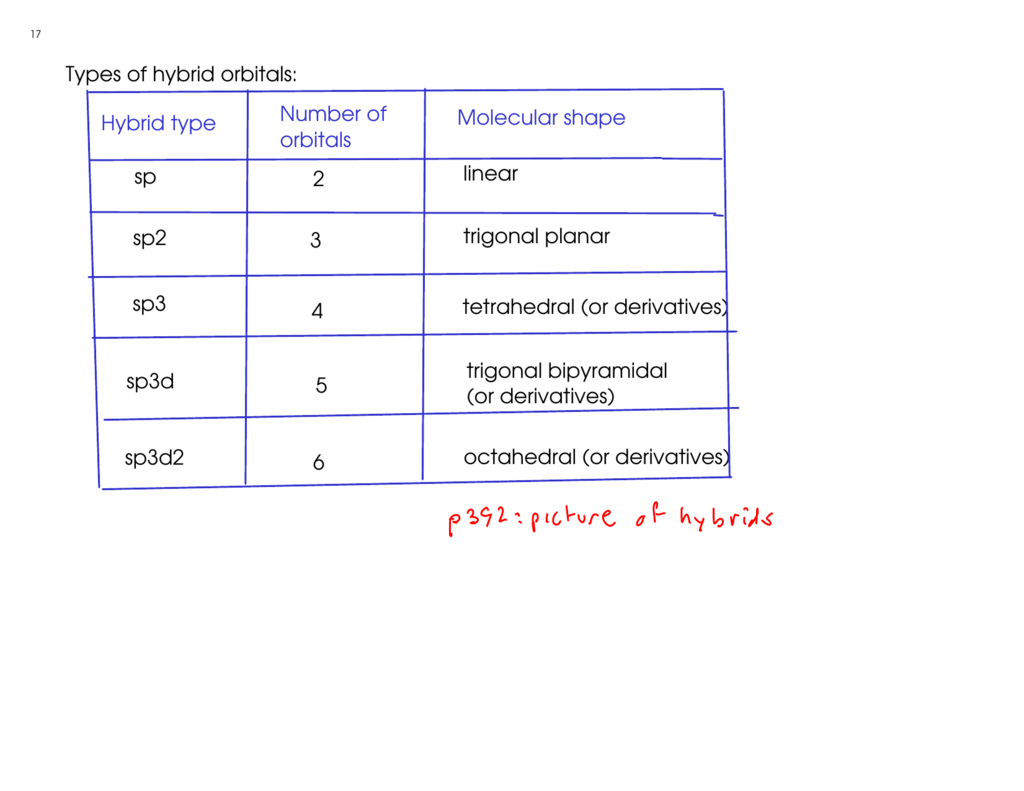
sp3d2 hybridization is the mixing of s, p and d atomic orbitals of the same electron shell to form sp3d2 hybrid orbitals. There, one s atomic orbital, three p atomic orbitals and two d atomic orbitals mix with each other. This mixing results in six hybrid orbitals of same size and shape but different from their orientation.
The sp3d2 hybrid orbitals are arranged in octahedral arrangement. These hybrid orbitals have 90o angles between two orbitals in the octahedral arrangement. The octahedral arrangement displays a square plane having four hybrid orbitals and the two remaining orbitals are oriented above and below of this square plane (perpendicular to this plane).
Example
Let us consider an example in order to understand the sp3d2 hybridization. Ex: SF6 molecule has an octahedral shape because the 3s, 3p and 3d atomic orbitals of the sulfur atom (S) are mixed to formsp3d2 hybrid orbitals.
Figure 01: Electronic structure of sulfur atom before and after Hybridization.
As shown in the above image, the hybridization results in six unpaired electrons that can participate in chemical bonding with six fluorine atoms. Most importantly, all the atomic orbitals involved in this hybridization are in the same electron shell (in above example, it is n=3 electron shell). Office 365 download free for mac.
What is d2sp3 Hybridization?
d2sp3 hybridization is the mixing of s and p atomic orbitals of the same electron shell with d orbitals of another electron shell to form d2sp3 hybrid orbitals. This hybridization results in six hybrid orbitals. These hybrid orbitals are arranged in an octahedral geometry.
Most importantly, in this hybridization, the d atomic orbitals come from a different electron shell (n-1 electron shell) whereas s and p atomic orbitals are of the same electron shell. Let us consider an example to understand this hybridization. Most of the metal ion complexes are composed of d2sp3 hybridized orbitals.
Example
For example, take Co(NH3)3+ complex.
Figure 02: Electronic structure of cobalt (Co) atom before and after Hybridization.
As shown in the above image, there are six empty hybrid orbitals in cobalt atom after hybridization. These empty orbitals can participate in coordination chemical bond formation with ligands (here ammonia ligands = NH3).
What are the Similarities Between sp3d2 and d2sp3 Hybridization?
Sp3d2 Hybridization Geometry
- Both sp3d2 and d2sp3 Hybridizations result in octahedral geometry.
- Both sp3d2 and d2sp3 Hybridization geometries have 90o angle between hybrid orbitals.
- Both sp3d2 and d2sp3 Hybridization result in six hybrid orbitals.
What is the Difference Between sp3d2 and d2sp3 Hybridization?
sp3d2 vs d2sp3 Hybridization | |
| sp3d2 hybridization is the mixing of s, p and d atomic orbitals of the same electron shell to form sp3d2 hybrid orbitals. | d2sp3 hybridization is the mixing of s and p atomic orbitals of the same electron shell with d orbitals of another electron shell to form d2sp3 hybrid orbitals. |
| Nomenclature | |
| sp3d2 hybridization forms sp3d2hybrid orbitals. | d2sp3 hybridization d2sp3 hybrid orbitals. |
| Type of Atomic Orbitals | |
| sp3d2 hybridization involves atomic orbitals of same electron shell. | d2sp3 hybridization involves atomic orbitals of two electron shells. |
| d Orbitals | |
| sp3d2 hybridization involves d atomic orbitals of n electron shell. | d2sp3 hybridization involves d atomic orbitals of n-1 electron shell. |
Summary – sp3d2 vs d2sp3 Hybridization
Sp3d2 Examples
sp3d2 hybridization and d2sp3 hybridization are confusing terms that are most of the times used interchangeably by mistake. These are different in many ways. The key difference between sp3d2 and d2sp3hybridization is that, sp3d2 hybridization involves atomic orbitals of same electron shell whereas d2sp3 hybridization involves atomic orbitals of two electron shells.
Download the PDF Version of sp3d2 vs d2sp3 Hybridization
You can download the PDF version of this article and use it for offline purposes as per citation note. Please download the PDF version here: Difference Between sp3d2 and d2sp3 Hybridisation
Sp3d2
Reference:
1.“8.2: Hybrid Atomic Orbitals.” Chemistry LibreTexts, Libretexts, 30 Aug. 2017. Available here
Sp3d2 Bonds
Related posts:
