(3 comments)
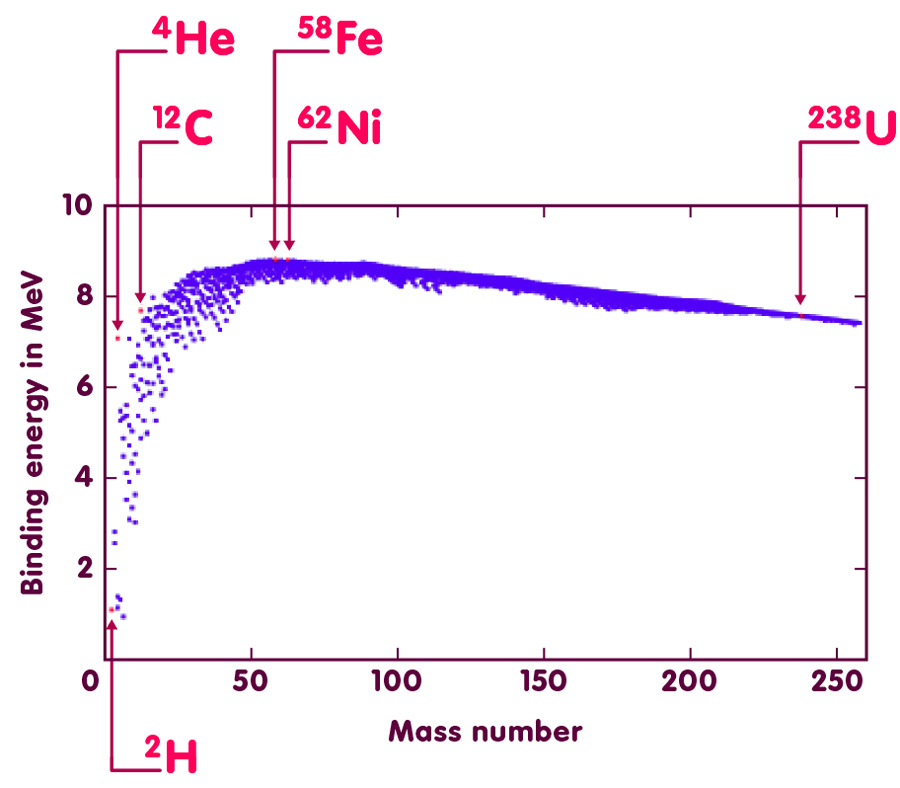
The binding energy of a nucleus is the energy that would be required to split apart each of its constituent protons and neutrons (nucleons). This energy is due to the forces that hold the nucleus together, which may be thought of as a balance between the attractive strong nuclear force and the electromagnetic force (which is repulsive between the positively-charged protons). Nuclear Binding Energy The energy required to break down a nucleus into its component nucleons is called the nuclear binding energy. 63 Cu + Energy 29 p + + 34 n o Nuclear binding energies are usually expressed in terms of kJ/mole of nuclei or MeV's/nucleon.
The binding energy of a nucleus is the energy that would be required to split apart each of its constituent protons and neutrons (nucleons). This energy is due to the forces that hold the nucleus together, which may be thought of as a balance between the attractive strong nuclear force and the electromagnetic force (which is repulsive between the positively-charged protons). The binding energy manifests itself in a mass difference between the nucleus and the individual nucleons ($E=mc^2$) and the difference in binding energy between different nuclei is the energy released in the process of nuclear fusion and fission.
The OECD's Nuclear Energy Agency publishes a compilation of nuclear data including recommended values for binding energies: mass.mas03 (G. Audi, A. H. Wapstra and C. Thibault, 'The Ame2003 atomic mass evaluation (II)', Nuclear PhysicsA729, 337–676 (2003).) The binding energy reported is an average per nucleon: $bar{E}_mathrm{bind} = E_mathrm{bind} / A$ where $A=N+Z$ is the total number of nucleons, the sum of the number of neutrons, $N$ and protons, $Z$.
A semi-emprical mass formula (SEMF) based on the George Gamow's so-called liquid drop model can be used to estimate mean binding energies:
$$bar{E}_mathrm{bind} = a_V - a_S A^{-1/3} - a_C Z^2 A^{-4/3} - A_A (A-2Z)^2A^{-2} pm delta A^{-3/2}$$
A brief description of these parameters is given below; more details can be found in standard textbooks on nuclear physics, and in outline on Wikipedia. Parameter values are taken from J. Free music download for mac air. W. Rohlf, Modern Physics from $alpha$ to $Z^0$, Wiley (1994).
| Parameter | Value /MeV | Description |
|---|---|---|
| $a_V$ | 15.75 | Volume term, due to the strong nuclear force |
| $a_S$ | 17.8 | Surface term, a correction to the volume term due to reduced interactions with nucleons at the surface of the nucleus |
| $a_C$ | 0.711 | Coulomb term, due to electrostatic repulsions between the protons |
| $a_A$ | 23.7 | Asymmetry term, explained by the need for nucleons to obey the Fermi exclusion principle |
| $pm delta$ | $pm 11.18$, $0$ | Pairing term, accounting for the effects of spin coupling. The positive sign applies to nuclei with $Z$ and $N$ both even; the negative sign to nuclei with $Z$ and $N$ both odd; otherwise this term is zero |
The following code generates the above plot of the recommended NEA binding energies and those derived using the SEMF approximation.
Related posts
Nuclear Binding Energy
The energy required to break down a nucleus into its component nucleons is called the nuclear binding energy.
63Cu + Energy ' height=11 src='NuclearArt/Arrow.gif' width=14 nosave> 29 p+ + 34 no

Nuclear binding energies are usually expressed in terms of kJ/mole of nuclei or MeV's/nucleon. Calculation of the nuclear binding energy involves the following three steps:
Determining the Mass DefectThe difference between the mass of a nucleus and the sum of the masses of the nucleons of which it is composed is called the mass defect. Three things need to be known in order to calculate the mass defect:
- the actual mass of the nucleus,
- the composition of the nucleus (number of protons and of neutrons),
- the masses of a proton and of a neutron.
- add up the masses of each proton and of each neutron that make up the nucleus,
- subtract the actual mass of the nucleus from the combined mass of the components to obtain the mass defect.
Nuclear Binding Energy Calculator
- Find the composition of the copper-63 nucleus and determine the combined mass of its components.
- Calculate the mass defect.
Copper has 29 protons and copper-63 also has (63 - 29) 34 neutrons. Doodly download for mac.
The mass of a proton is 1.00728 amu and a neutron is 1.00867 amu.
The combined mass is calculated:
or
63.50590 amu
Conversion of Mass Defect into Energy
To convert the mass defect into energy:

- Convert the mass defect into kilograms (1 amu = 1.6606 x 10-27 kg)
- Convert the mass defect into its energy equivalent using Einstein's equation.
Nuclear Binding Energy Example
Determine the binding energy of the copper-63 atom.- Convert the mass defect (calculated in the previous example) into kg.

- Convert this mass into energy using DE = Dmc2, where c = 2.9979 x 108 m/s.
Nuclear Binding Energy Per Nucleon
Expressing Nuclear Binding Energy as Energy per Mole of Atoms, or as Energy per Nucleon
The energy calculated in the previous example is the nuclear binding energy. However, nuclear binding energy is often expressed as kJ/mol of nuclei or as MeV/nucleon.
- To convert the energy to kJ/mol of nuclei we will simply employ the conversion factors for converting joules into kilojoules (1 kJ = 1000 J) and for converting individual particles into moles of particles (Avogadro's Number).
- To convert the binding energy to MeV (megaelectron volts) per nucleon we will employ the conversion factor for converting joules into MeV (1 MeV = 1.602 x 10-13 J) and the number of nucleons (protons and neutrons) which make up the nucleus.
